Optimisation techniques are used for the design of novel forced-periodic reactors in which there is the integration of catalytic
reaction, adsorptive separation and direct #uid to solid heat exchange within a single unit operation. For such con"gurations,
dynamic operation is utilised to generate favourable temperature (catalyst activity) pro"les, and, through in situ separation, to provide
reaction enhancement and the enriched recovery of the primary product(s). The work thus involves aspects of thermal and
concentration swing adsorption, reverse (or bidirectional) #ow reactor operation, and thermal regeneration. The design methodology
is based on fully discretised mathematical models, upon which periodic constraints are imposed to yield direct cyclic steady state
solutions. The models are then formulated as non-linear programming problems to yield optimal operating schedules and conditions.
Models are developed for general endothermic and equilibrium limited reaction schemes; as a case study, speci"c consideration is
given to the dehydrogenation of methylcyclohexane to toluene over and admixture of Pt}Al2O3catalyst and zeolite 5A adsorbent.
When compared to an equivalent and optimally operated adiabatic plug #ow reactor, design and operating conditions are calculated
for both single and multistage con"gurations in which there are signi"cant improvements in reactant conversion, with the additional
bene"t of the bulk separation of the product species. These qualities of the hybrid reactors are attained for energy inputs (i.e. feed
pre-heat requirements) which do not exceed that of the equivalent steady #ow reactor. The results also predict larger improvements in
reactor performance when there is greater #exibility in the way in which heat and material are introduced into the reactor systems. For
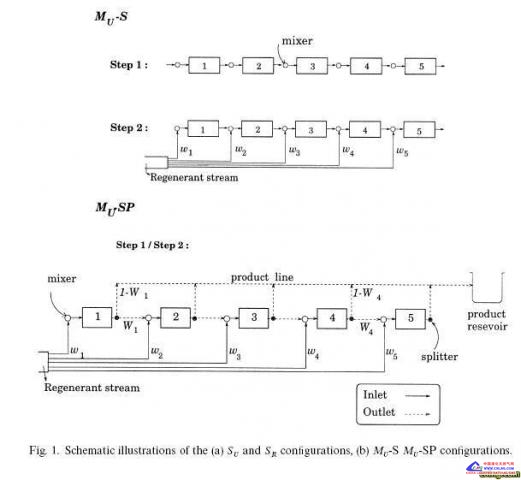
example, for a "ve-stage con"guration involving mixed series}parallel connections of the stages, and the disproportional splitting of
feed streams to each stage, a conversion of 59% is calculated for the production of 0.02 mol/m2 s toluene (cf. 23% for an equivalent
reactor), with 72% recovery of the toluene in a near pure form (inert-free basis).